Platinum-Cell Conductivity Probe User Manual
Order Code: CONPT-BTA
The Platinum-Cell Conductivity Probe can be used to measure either solution conductivity or total ion concentration of aqueous and non-aqueous samples in the field or in the laboratory. It has an epoxy body and platinum cell electrodes to provide accuracy and precision in a rugged body. Even though it does not identify specific ions, it quickly determines the total concentration of ions in a sample. It can be used to perform a wide variety of tests or experiments to determine the total dissolved ion concentrations:
- Allow students to quantitatively see the difference between the ionic and molecular nature of electrolytes in aqueous and non-aqueous solution. This can include differences in strength of weak acids and bases, or the number of ions that an ionic substance dissociates into per formula unit.
- Confirm the direct relationship between conductivity and ion concentration in an aqueous or non-aqueous solution. Concentrations of unknown samples can then be determined.
- Monitor the rate of a chemical reaction in which dissolved ions and solution conductivity varies with time due to an ionic species being consumed or produced.
- Determine the rate at which an ionic species diffuses through a membrane, such as dialysis tubing.
Note: Vernier products are designed for educational use. Our products are not designed nor are they recommended for any industrial, medical, or commercial process such as life support, patient diagnosis, control of a manufacturing process, or industrial testing of any kind.
Compatible Software
Choose a platform below to see its compatibility requirements.LabQuest
Interface LabQuest App LabQuest 3 Full support LabQuest 2 Full support LabQuest Full support Computers
Software Interface Graphical Analysis Graphical Analysis (Web App) Logger Pro (discontinued) Logger Lite (discontinued) LabQuest Mini Full support Full support Full support Full support LabQuest 3 Full support Full support Full support Incompatible LabQuest 2 Full support Full support Full support Full support LabQuest Stream Full support 1 Full support 1 Partial support 2 Full support 1 Go!Link Full support Full support Full support Full support LabQuest Full support Full support Full support Full support LabPro Incompatible Incompatible Full support Full support Compatibility Notes
Chromebook
Software Interface Graphical Analysis (Web App) LabQuest Mini Full support LabQuest 3 Full support LabQuest 2 Full support LabQuest Stream Full support 1 Go!Link Full support LabQuest Full support Compatibility Notes
iOS
Software Interface Graphical Analysis Graphical Analysis GW LabQuest Stream Full support Full support LabQuest 3 Full support 1 Full support 1 LabQuest 2 Full support 1 Full support 1 Compatibility Notes
Android
Software Interface Graphical Analysis Graphical Analysis GW LabQuest Stream Full support Partial support 1 LabQuest 3 Full support 2 Full support 2 LabQuest 2 Full support 2 Full support 2 Compatibility Notes
Arduino
Software Interface Arduino Vernier Arduino® Interface Shield Full support LabVIEW
Software Interface NI LabVIEW SensorDAQ Full support Vernier myDAQ Adapter Full support 1 Go!Link Full support LabQuest Mini Full support LabQuest Stream Full support LabQuest 3 Full support LabQuest 2 Full support LabQuest Full support Compatibility Notes
Texas Instruments
Software Interface EasyData DataMate TI-84 SmartView DataQuest TI-Nspire Software EasyLink Full support 1 Incompatible Full support 2 Full support Full support 2 CBL 2 Full support 3 Full support 3 4 Incompatible Incompatible Incompatible LabPro Full support 3 Full support 3 4 Incompatible Incompatible Incompatible TI-Nspire Lab Cradle Incompatible Incompatible Incompatible Full support Full support Compatibility Notes
Quick Start
- Plug the sensor into the interface (LabQuest 3, LabQuest Mini, etc.).
- Connect the interface to your device.
- If using USB, connect to the USB port on your computer.
- If using Bluetooth® wireless technology, click your interface type and then select your device.
- Prepare for data collection:
- Vernier Graphical Analysis®: Launch the app, if necessary, and click Sensor Data Collection.
- LabQuest® App: Choose New from the File menu.
The software will identify the sensor and load a default data-collection setup. You are now ready to collect data.
Need Additional Information?
Visit the following link:
Using the Product
Taking Measurements with the Platinum-Cell Conductivity Probe
- For best results, condition the electrode in a standard solution for approximately five minutes prior to use.
- Rinse the tip of the Platinum-Cell Conductivity Probe with distilled water. Optional: Blot the inside and outside of the electrode cell dry to avoid water droplets diluting or contaminating the sample to be tested.
- Insert the tip of the probe into the sample to be tested. Important: Be sure the electrode surfaces in the elongated cell are completely submerged in the liquid and that there are no bubbles around the electrode surface.
- Wait for the reading on your data-collection device to stabilize. This should take no more than 5 to 10 seconds. Note: Do not completely submerge the sensor. The handle is not waterproof.
- Rinse the tip of the probe with distilled water before taking another measurement.
Using the Platinum-Cell Conductivity Probe with Other Vernier Sensors
Some combinations of sensors interfere with each other when placed in the same solution. The degree of interference depends on many factors, including which combination of sensors is being used, which interface is being used, and others.
Relationship between Conductivity and TDS
Because there is a nearly linear relationship between conductivity and concentration of a specific ion or salt, the Platinum-Cell Conductivity Probe can be used to determine the concentration of an ion. A curve similar to the one shown in Figure 1 can be obtained if you prepare or purchase standard solutions (solutions with known concentra¬tions). Note in this figure that the 2:1 ratio between conductivity in µS/cm and TDS concentration in mg/L.
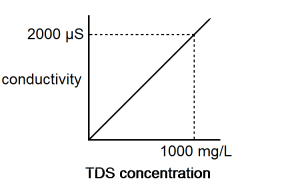
Even though total dissolved solids is often defined in terms of this 2:1 ratio, it should be understood that a TDS reading of 500 mg/L can have a different meaning in a sample that is mostly NaCl than in another sample that is composed primarily of hard water ions such as Ca2+ and HCO3–. The relationship between conductivity and sodium chloride concentration is approximately a 2:1 ratio and is very nearly a direct relationship. Table 1 shows the relationship of sodium chloride concentration in mg/L to TDS and conductivity.
| Table 1 | ||
|
Sodium
chloride concentration |
Total
dissolved solids (TDS) |
Conductivity
|
|
1.0 |
1.1 |
2.2 |
|
5.0 |
5.4 |
10.8 |
|
10 |
10.7 |
21.4 |
|
20 |
21.4 |
42.7 |
|
50 |
52.5 |
105 |
|
100 |
105 |
210 |
|
150 |
158 |
315 |
|
200 |
208 |
415 |
|
500 |
510 |
1020 |
|
1000 |
995 |
1990 |
|
1500 |
1465 |
2930 |
|
2000 |
1930 |
3860 |
|
5000 |
4482 |
8963 |
|
10250 |
9000 |
18000 |
Calibrating the Sensor
Conductivity Probe for most experiments. Each probe is programmed with a custom calibration prior to shipping. The factory calibration is set while the probe is at room temperature, with the temperature compensation at 2%.
However, if your experimental application requires more accurate readings or if you are testing without temperature compensation, you should calibrate your sensor. The Platinum-Cell Conductivity Probe can be easily calibrated at two known levels, using any of the Vernier data-collection programs. The calibration units can be µS/cm, dS/cm, mg/L, ppm, or ppt. For best results, it is recommended that the two-point calibration be performed using two standard solutions that bracket the expected range of conductivity or concentration values you will be testing. For example, if you expect to measure conductivity in the range of 600 mg/L to 1000 mg/L (TDS), you may want to use a standard solution that is 500 mg/L for one calibration point and another standard that is 1000 mg/L for the second calibration point.
To Calibrate
- Make sure the temperature compensation switch is set to the desired position.
- Initiate the calibration procedure in the software.
- First calibration point: Place the Platinum-Cell Conductivity Probe into a known standard solution. Be sure the entire elongated hole with the electrode surfaces is submerged in the solution and that there are no bubbles along the electrode surface. Wait for the displayed voltage to stabilize. Enter the value of the standard solution in the appropriately chosen units for Reading 1. Click Keep. Note: Performing a zero point calibration is not recommended with conductivity sensors. It is preferred that you use a low standard calibration standard instead of a zero point. This is particularly important if you plan to take measurements below 200 µS/cm where the low calibration point is most critical.
- Second calibration point: Place the Platinum-Cell Conductivity Probe into a different standard solution. Be sure the entire elongated hole with the electrode surfaces is submerged in the solution and that there are no bubbles along the electrode surface. Wait for the displayed voltage to stabilize. Enter the value of the standard solution for Reading 2. Click Keep.
- If you want to use the calibration for the current session only, click Done to complete the calibration process. To save the calibration onto the sensor, click the storage tab and save to sensor.
- Click Done or tap OK to complete the calibration process.
Standard Calibration Solutions
If you choose to calibrate the Platinum-Cell Conductivity Probe, you will want accurate standard solutions. Prepare solutions that are appropriate for the samples and conductivity range of your experiment. If you are testing aqueous samples, Vernier sells two Conductivity Standards appropriate for the range of the Platinum-Cell Conductivity Probe. These standards are available in 500 mL bottles. The order codes are
|
Conductivity Standard Solution (Low) (150 µS/cm) |
CON-LST |
|
Conductivity Standard Solution (Middle) (1413 µS/cm) |
CON-MST |
If you are testing non-aqueous samples, prepare standard solutions of known conductivity that contain similar compounds to those being tested.
Temperature Compensation
The Platinum-Cell Conductivity Probe has two temperature compensation settings: 0% and 2%. The 2% setting is designed for most aqueous salt solutions.
If you select the 2% setting, the sensor reading is automatically temperature compensated between temperatures of 5 and 35°C. Note that the temperature of a solution is being read by a thermistor that is embedded in the electrode. Readings are automatically referenced to a conductivity value at 25°C; therefore, the Platinum-Cell Conductivity Probe will give the same conductivity reading in a solution that is at 15°C as it would if the same solution were warmed to 25°C. This means you can calibrate your probe in the lab, and then use these stored calibrations to take readings in colder (or warmer) water in a lake or stream.
If you are testing a non-aqueous solution and want temperature-compensated readings, you will have to perform your own temperature standardization curve or research the value. Some values are presented in Table 2. If the 0% temperature compensation setting is selected, the probe is not temperature compensated and you will notice a change in the conductivity reading as temperature changes, even though the actual ion concentration does not change. This setting allows you to investigate conductivity as a function of temperature.
| Table 2 | ||
|
Sample |
Conductivity |
% change/°C |
|
Ultrapure Water |
0.055 |
4.55 |
|
Drinking Water |
50–500 |
2.00 |
|
0.1% NaCl |
1990 |
2.12 |
|
0.03% NaOH |
1780 |
1.72 |
|
20% Acetic Acid |
1600 |
1.56 |
|
5% NaOH |
223,000 |
1.72 |
|
10% HCl |
700,000 |
1.32 |
Sample of typical temperature coefficients and corresponding conductivities
Specifications
|
Range |
0 to 2000 µS/cm (0 to 1000 mg/L ) |
|
Body description |
epoxy body, 2-cell platinum element |
|
Accuracy using factory calibration |
±40 µS/cm |
|
Accuracy using custom calibration |
±10 µS/cm |
|
Response time |
95% of full-scale in 5 seconds |
|
Temperature compensation |
Optional: 2% from 5 to 35°C or none |
|
Temperature range |
0 to 80°C |
|
Cell constant |
1.0 cm-1 |
|
Shaft dimensions |
12 mm OD × 120 mm length |
Care and Maintenance
- When you have finished using the Platinum-Cell Conductivity Probe, simply rinse it off with distilled water and blot it dry using a paper towel or lab wipe. The probe can then be stored dry.
- If the probe cell surface is contaminated, soak it in water with a mild detergent for 15 minutes. Then soak it in a dilute acid solution (0.1 M hydrochloric acid or 0.5 M acetic acid works well) for another 15 minutes. Then rinse it well with distilled water and blot dry. Important: Avoid scratching the inside electrode surfaces of the elongated cell.
- Do not wrap the cable tightly around the sensor for storage. Repeatedly doing so can irreparably damage the wires and is not covered under warranty.
How the Sensor Works
The Vernier Platinum-Cell Conductivity Probe measures the ability of a solution to conduct an electric current between two electrodes. In solution, the current flows by ion transport. Therefore, an increasing concentration of ions in the solution will result in higher conductivity values.
The Platinum-Cell Conductivity Probe is actually measuring conductance, defined as the reciprocal of resistance. When resistance is measured in ohms, conductance is measured using the SI unit, siemens (formerly known as a mho). Since the siemens is a very large unit, aqueous samples are commonly measured in microsiemens, or µS.
Even though the Platinum-Cell Conductivity Probe is measuring conductance, we are often interested in finding conductivity of a solution. Conductivity, C, is found using the following formula:
C = G • kc
where G is the conductance, and kc is the cell constant. The cell constant is determined for a probe using the following formula:
kc = d/A
where d is the distance between the two electrodes, and A is the area of the electrode surface.
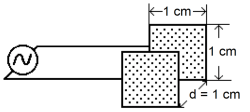
For example, the cell in Figure 2 has a cell constant:
kc = d/A = 1.0 cm/1.0 cm2= 1.0 cm–1
The conductivity value is found by multiplying conductance and the cell constant. Because the Vernier Platinum-Cell Conductivity Probe also has a cell constant of 1.0 cm–1, its conductivity and conductance have the same numerical value. For a solution with a conductance value of 1000 µS, the conductivity, C, would be:
C = G • kc = (1000 µS) × (1.0 cm–1) = 1000 µS/cm
A potential difference is applied to the two probe electrodes in the Platinum-Cell Conductivity Probe. The resulting current is proportional to the conductivity of the solution. This current is converted into a voltage. Alternating current is supplied to prevent the complete ion migration to the two electrodes. With each cycle of the alternating current, the polarity of the electrodes is reversed, which in turn reverses the direction of ion flow. This very important feature of the Platinum-Cell Conductivity Probe prevents most electrolysis and polarization from occurring at the electrodes. Thus, the solutions that are being measured for conductivity are not fouled. It also greatly reduces redox products from forming on the platinum cell electrodes.
Troubleshooting
For troubleshooting and FAQs, see www.vernier.com/til/3477
Repair Information
If you have watched the related product video(s), followed the troubleshooting steps, and are still having trouble with your Platinum-Cell Conductivity Probe, contact Vernier Technical Support at support@vernier.com or call 888-837-6437. Support specialists will work with you to determine if the unit needs to be sent in for repair. At that time, a Return Merchandise Authorization (RMA) number will be issued and instructions will be communicated on how to return the unit for repair.
Accessories/Replacements
| Item | Order Code |
|---|---|
|
CB-USB-MICRO |
|
|
CON-LST |
|
|
CON-MST |
|
|
CON-HST |
|
|
ESUP |
|
|
MSTIR |
Warranty
Warranty information for this product can be found on the Support tab at www.vernier.com/CONPT-BTA/#support
General warranty information can be found at www.vernier.com/warranty
Contact Support
Fill out our online support form or call us toll-free at 1-888-837-6437.

