Under Pressure – The Inverse Relationship between Pressure and Volume
Experiment #14 from Real-World Math with Vernier
- Education Level
- High School
Introduction
Let’s take a sample of air in a closed container, and keep it at room temperature. If you change the volume of the container, what will happen to the air pressure inside? You can feel this by squeezing a small balloon in your hand. As the balloon gets smaller, you have to push harder. That is, as the volume decreases, the pressure increases. Two quantities that change in this way could be inversely related. If pressure and volume are inversely related, even if both quantities change, then their product stays the same.
Suppose that x and y represent the quantities that are inversely related. Then
where k is a constant in both equations. Maybe you can think of some other quantities that also behave this way. For air and other gases, this relation has a name: Boyle’s law.
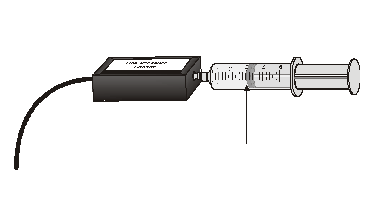
In this activity, you will use a pressure sensor to investigate the relationship between pressure and volume for air contained within a closed syringe.
Objectives
- Record pressure versus volume data for a sample of air.
- Fit an inverse function model to the data.
- Re-plot the data using linearization.
Sensors and Equipment
This experiment features the following sensors and equipment. Additional equipment may be required.
Ready to Experiment?
Ask an Expert
Get answers to your questions about how to teach this experiment with our support team.
- Call toll-free: 888-837-6437
- Chat with Us
- Email support@vernier.com
Purchase the Lab Book
This experiment is #14 of Real-World Math with Vernier. The experiment in the book includes student instructions as well as instructor information for set up, helpful hints, and sample graphs and data.


