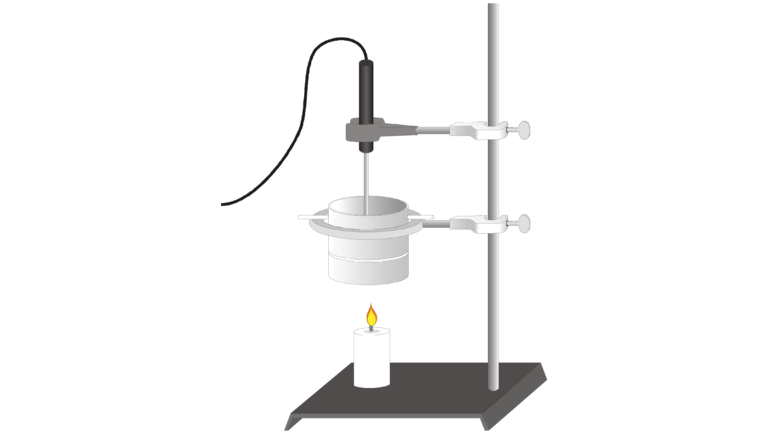
Introduction
Energy content is an important property of fuels. This property helps scientists and engineers determine the usefulness of a fuel. Energy content is the amount of heat produced by the burning of 1 gram of a substance, and is measured in joules per gram (J/g).
You can determine energy content of a fuel by burning an amount of the fuel and capturing the heat released in a known mass of water in a calorimeter. If you measure the initial and final temperatures, the energy released can be calculated using the equation
where H = heat energy absorbed (in J), Δt = change in temperature (in °C), m = mass (in g), and Cp = specific heat capacity (4.18 J/g°C for water). Dividing the resulting energy value by grams of food burned gives the energy content (in J/g).
Objectives
In this experiment, you will
- Measure temperature.
- Analyze data.
- Use a balance.
- Determine energy content.
- Compare the energy content of different fuels.
Sensors and Equipment
This experiment features the following sensors and equipment. Additional equipment may be required.
Option 1

Ready to Experiment?
Ask an Expert
Get answers to your questions about how to teach this experiment with our support team.
- Call toll-free: 888-837-6437
- Chat with Us
- Email support@vernier.com
Purchase the Lab Book
This experiment is #9 of Physical Science with Vernier. The experiment in the book includes student instructions as well as instructor information for set up, helpful hints, and sample graphs and data.


