Introduction
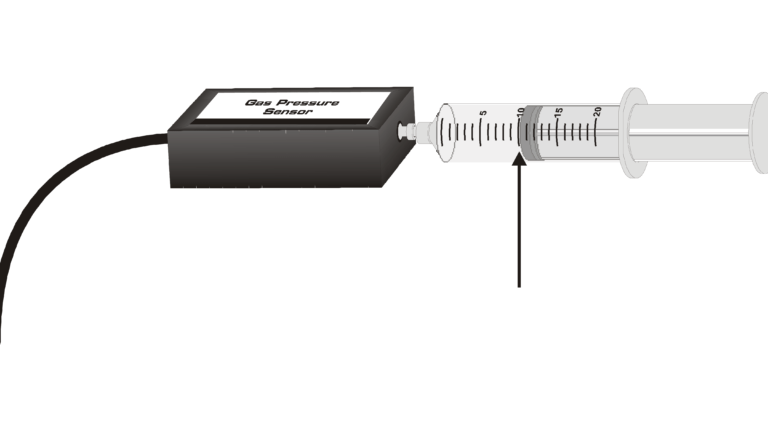
In this simple experiment, you will use a Gas Pressure Sensor and a gas syringe to study the relationship between gas pressure and volume. Temperature and amount of gas will be kept constant. The results will be expressed in words, in a table, with a graph, and with a mathematical equation. These are four methods commonly used by scientists to communicate information.
This experiment is similar to one first done by Robert Boyle in 1662—without the use of a computer, of course. The relationship you will discover is known as Boyle’s law.
Objectives
In this experiment, you will
- Use a Gas Pressure Sensor and a gas syringe to measure the pressure of an air sample at several different volumes.
- Make a table of the results.
- Graph the results.
- Predict the pressure at other volumes.
- Describe the relationship between gas pressure and volume with words and with a mathematical equation.
Sensors and Equipment
This experiment features the following sensors and equipment. Additional equipment may be required.
Option 1

Option 2

Ready to Experiment?
Ask an Expert
Get answers to your questions about how to teach this experiment with our support team.
- Call toll-free: 888-837-6437
- Chat with Us
- Email support@vernier.com
Purchase the Lab Book
This experiment is #30 of Physical Science with Vernier. The experiment in the book includes student instructions as well as instructor information for set up, helpful hints, and sample graphs and data.

