Introduction
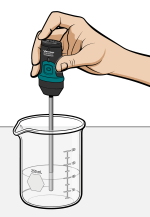
Cold packs are used to treat sprained ankles and similar injuries. A cold pack is typically made of a thin plastic inner bag containing water. That bag, in turn, is surrounded by a heavier plastic bag containing a solid substance. When the pack is twisted, the inner bag breaks and releases the water. As the solid substance dissolves in the water, energy is absorbed and the resulting mixture gets colder.
In this experiment, you will first determine temperature changes as several different solid substances dissolve in water. You will then develop and test a plan for making the best cold pack using 3.0 grams of one of the substances and the best amount of water.
Objectives
In this experiment, you will
- Use a Temperature Probe to measure temperature.
- Determine temperature changes as solid substances dissolve in water.
- Design and test a plan for making the best cold pack.
- Report your results.
Sensors and Equipment
This experiment features the following sensors and equipment. Additional equipment may be required.
Option 1

Ready to Experiment?
Ask an Expert
Get answers to your questions about how to teach this experiment with our support team.
- Call toll-free: 888-837-6437
- Chat with Us
- Email support@vernier.com
Purchase the Lab Book
This experiment is #16 of Middle School Science with Vernier. The experiment in the book includes student instructions as well as instructor information for set up, helpful hints, and sample graphs and data.


