Introduction
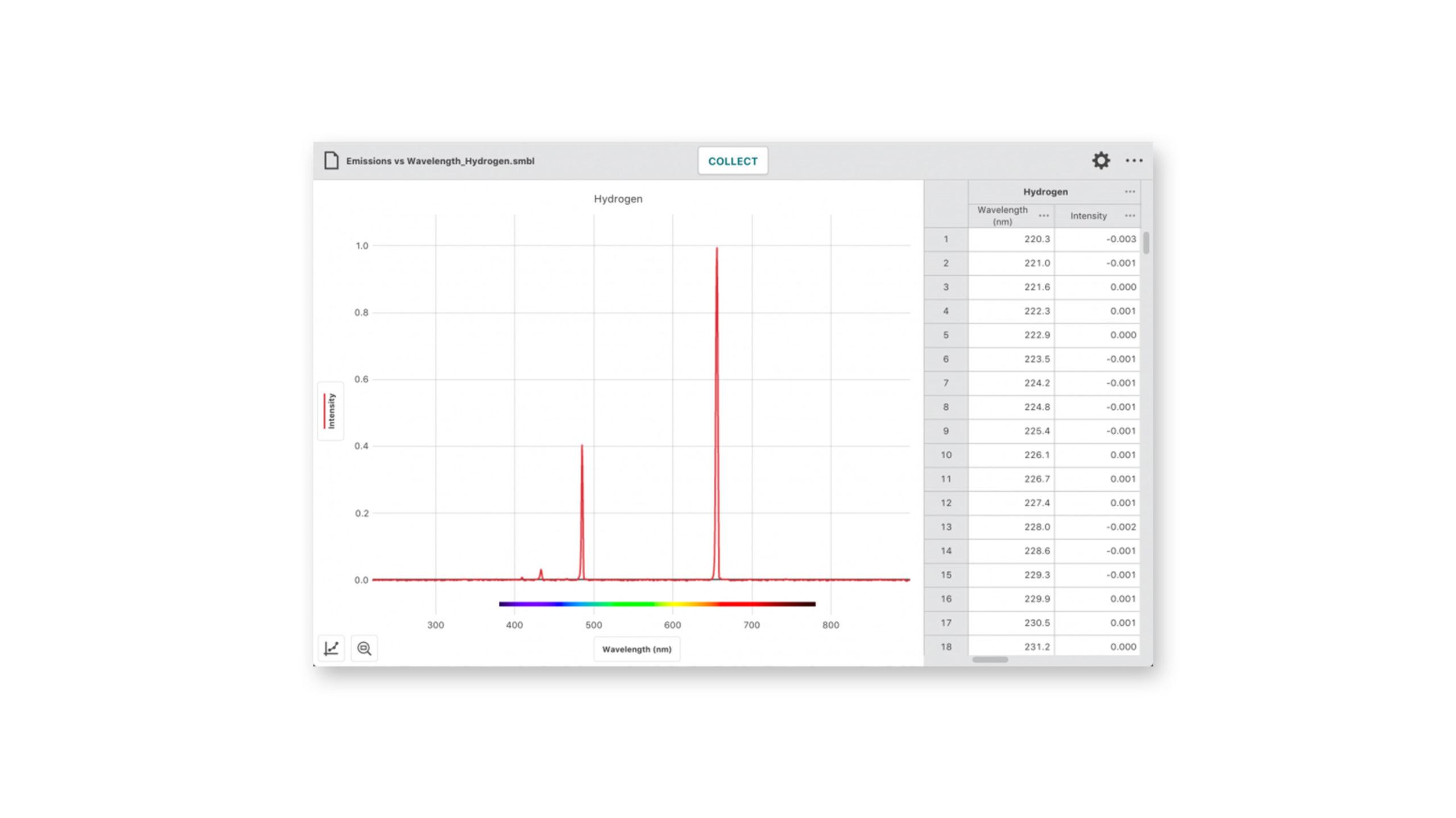
Every element on the periodic table produces a unique emission spectrum when excited. When exposed to heat or electromagnetic energy, some of the electrons in the atom are elevated to higher energy orbitals. This results in an unstable state for the atom. To return to a lower, more stable energy state, the excited electrons return to a lower energy level by emitting specific quantities of energy in the electromagnetic spectrum, some of which may be visible as bands of various colors.
Since the CSI techs couldn’t identify any toxic chemicals in the sports drink, they suspect toxic metal poisoning. You will use flame testing to check for the presence of metal ions in a sports drink.
Also, you will calculate the energy of the photons emitting while the electrons are returning to lower energy levels. The formula to calculate the energy of photons is
E = hν
where h = Planck’s constant, 6.63 × 10–34 J-s, and ν is the frequency of light emitted.
Since the spectrum you capture will provide λ, the wavelength of the light emitted, you’ll need to convert the wavelength to frequency. To do this, you will use the wave equation
c = λν
where c = the speed of light, 3.00 × 108 m/s, and λ is the wavelength you will read from the graph of the emission spectrum.
Objectives
- Carry out an investigation to capture emission spectra of metal ions in solution.
- Analyze data to identify the color and wavelength of the most prominent peaks in the spectrum of each metal ion.
- Use mathematical representations to calculate the energy of the photons producing the peaks in the spectra.
- Interpret data to identify the unknown metal in a sports drink by comparing emission spectra from known metals.
Sensors and Equipment
This experiment features the following sensors and equipment. Additional equipment may be required.
Correlations
Teaching to an educational standard? This experiment supports the standards below.
- International Baccalaureate (IB) 2025/Chemistry
- Structure 1.3.1—Emission spectra are produced by atoms emitting photons when electrons in excited states return to lower energy levels.
Ready to Experiment?
Ask an Expert
Get answers to your questions about how to teach this experiment with our support team.
- Call toll-free: 888-837-6437
- Chat with Us
- Email support@vernier.com
Purchase the Lab Book
This experiment is #12 of Forensic Chemistry Experiments. The experiment in the book includes student instructions as well as instructor information for set up, helpful hints, and sample graphs and data.