Introduction
SOUTH PAINTER, Tuesday: It was a normal Monday morning at GlobalTech Industries until the mail boy discovered project manager
Patrick Marchand dead in his cubicle, head on his desk. Mr. Marchand had died while writing an email, in a room full of people hard at
work. An early examination of the crime scene yielded no clues.
Mr. Marchand was known to have a serious heart condition, and many signs pointed to cardiac arrest as the cause of his death. However, as police canvassed the office space, the distinct odor of bitter almonds was detected, and a vial containing a small amount of an unknown chemical was found discarded in a communal trash can.
Objectives
- Use Beer’s law to determine the concentration of simulated iron(III) thiocyanate (FeSCN2+) in an unknown solution.
- Use colorimetry to determine the concentration of a colored species in a solution.
- Use a linear relationship to model the data (Beer’s law).
- Learn the importance of carefully prepared standards.
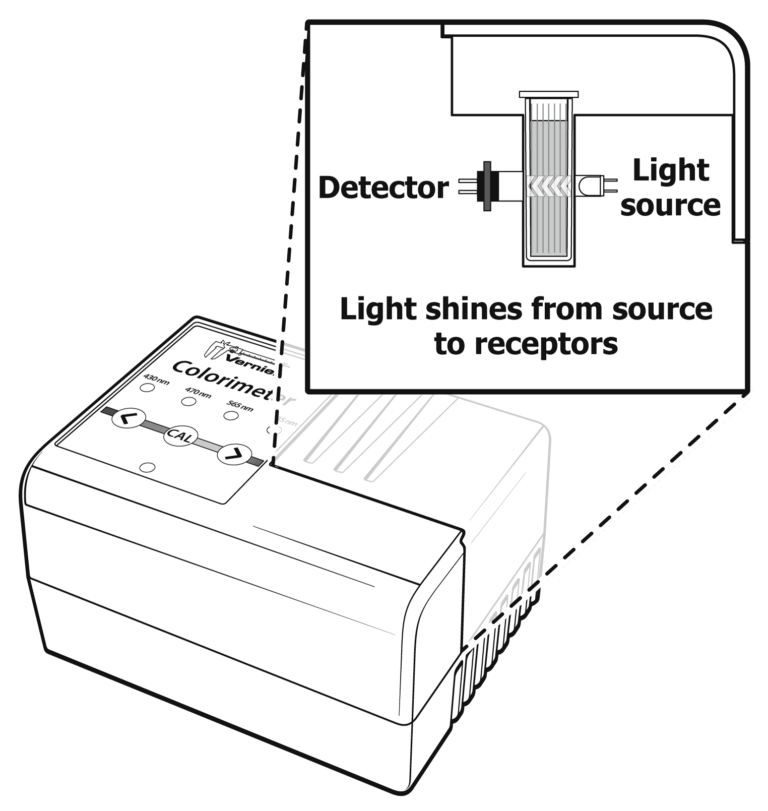
Sensors and Equipment
This experiment features the following sensors and equipment. Additional equipment may be required.
Option 1

Option 2

Ready to Experiment?
Ask an Expert
Get answers to your questions about how to teach this experiment with our support team.
- Call toll-free: 888-837-6437
- Chat with Us
- Email support@vernier.com
