Introduction
Do you ever wonder why you can only blow so much air into a balloon before the balloon bursts? Or why a volcano erupts and shoots gases, dust, and molten rock high up into the air? This activity will allow you to explore pressure changes similar to these in the safety of your own classroom! Be sure to wear your safety goggles whenever you work with chemical reactions — this activity is a BLAST!
Objectives
- Record what happens to the air pressure when you combine vinegar and baking soda in a plastic water bottle.
- Find out what happens when you mix different amounts of vinegar and baking soda together.
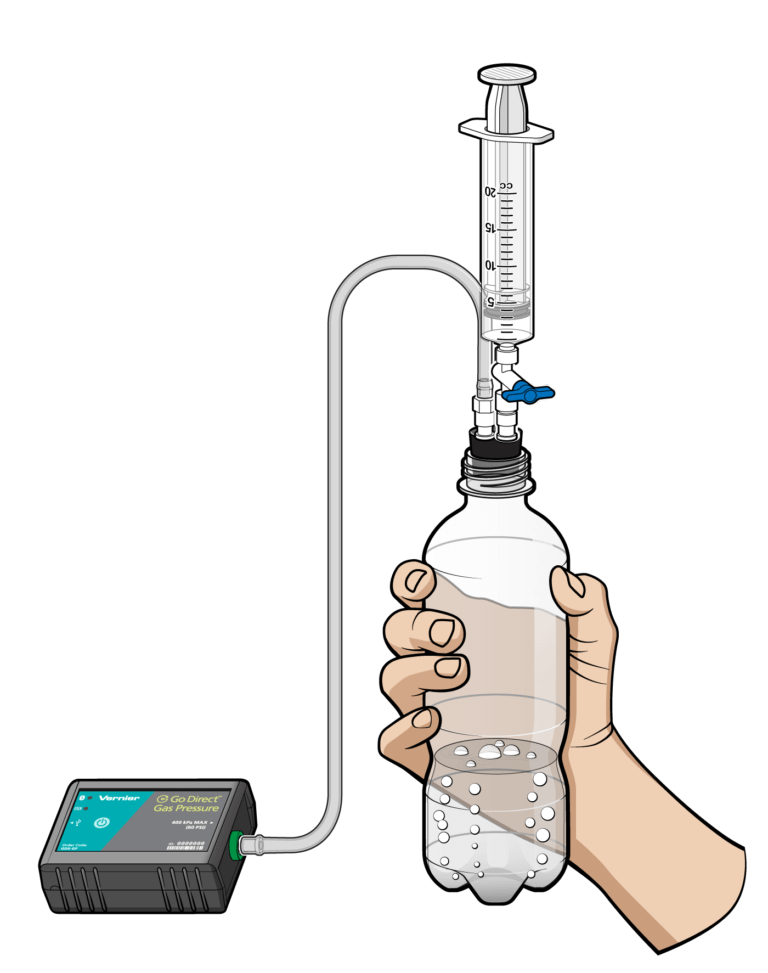
Sensors and Equipment
This experiment features the following sensors and equipment. Additional equipment may be required.
Ready to Experiment?
Ask an Expert
Get answers to your questions about how to teach this experiment with our support team.
- Call toll-free: 888-837-6437
- Chat with Us
- Email support@vernier.com
Purchase the Lab Book
This experiment is #2 of Investigating Gas Pressure. The experiment in the book includes student instructions as well as instructor information for set up, helpful hints, and sample graphs and data.


