Fractional Distillation
Experiment #8 from Chemistry with Vernier
- Education Level
- High School
- Subject
- Chemistry
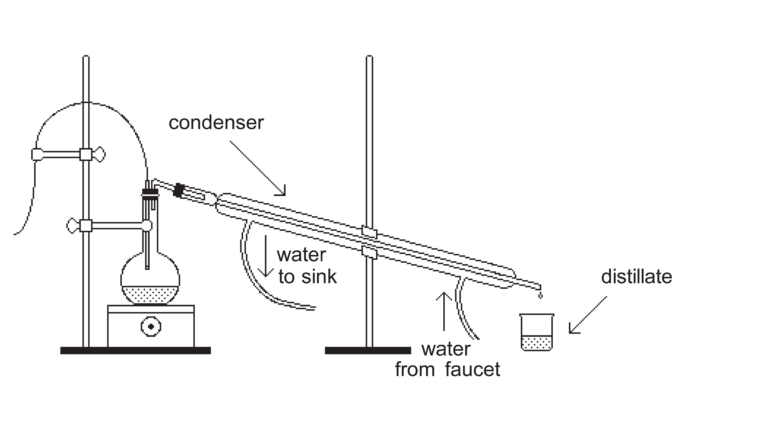
Introduction
An example of a simple distillation is the separation of a solution of salt and water into two separate pure substances. When the salt water solution is heated to boiling, water vapor from the mixture reaches the condenser and the cold water circulating around the inside tube causes condensation of water vapor into droplets of liquid water. The liquid water is then collected at the lower end of the condenser. The non-volatile salt remains in the flask.
In this experiment, the initial mixture you distill contains two volatile liquids: ethanol and water. In this distillation, both of the liquids will evaporate from the boiling solution. Ethanol and water have normal boiling temperatures of 79°C and 100°C, respectively. One objective of the experiment is to observe what happens when a liquid-liquid mixture is heated and allowed to boil over a period of time. Throughout the distillation, volumes of distillate, called fractions, will be collected. The percent composition of ethanol and water in each fraction will be determined from its density. Water has a density of 1.00 g/cm3 (at 20°C) and ethanol has a density of 0.79 g/cm3 (at 20°C). The fractions you collect will have densities in this range.
Objectives
In this experiment, you will
- Observe what happens when a liquid-liquid mixture is heated and allowed to boil over a period of time.
- Determine percent composition of ethanol and water in the fraction from its density.
Sensors and Equipment
This experiment features the following sensors and equipment. Additional equipment may be required.
Option 1

Correlations
Teaching to an educational standard? This experiment supports the standards below.
- International Baccalaureate (IB) 2025/Chemistry
- Structure 1.1.2—The kinetic molecular theory is a model to explain physical properties of matter (solids, liquids and gases) and changes of state.
Ready to Experiment?
Ask an Expert
Get answers to your questions about how to teach this experiment with our support team.
- Call toll-free: 888-837-6437
- Chat with Us
- Email support@vernier.com
Purchase the Lab Book
This experiment is #8 of Chemistry with Vernier. The experiment in the book includes student instructions as well as instructor information for set up, helpful hints, and sample graphs and data.


