Rate Law Determination of the Crystal Violet Reaction
Experiment #30 from Chemistry with Vernier
- Education Level
- High School
- Subject
- Chemistry
Introduction
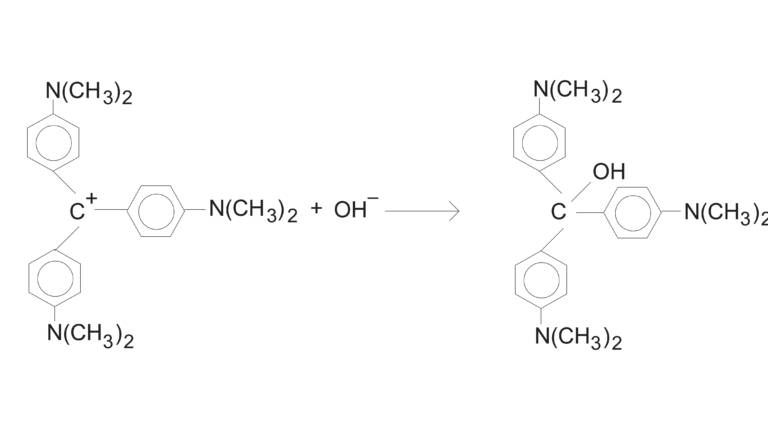
In this experiment, you will observe the reaction between crystal violet and sodium hydroxide. One objective is to study the relationship between concentration of crystal violet and the time elapsed during the reaction. A simplified version of the equation is:
The rate law for this reaction is in the form: rate = k[CV+]m[OH–]n, where k is the rate constant for the reaction, m is the order with respect to crystal violet (CV+), and n is the order with respect to the hydroxide ion. Because the hydroxide ion concentration is more than 1000 times as large as the concentration of crystal violet, [OH–] will not change appreciably during this experiment. Thus, you will find the order with respect to crystal violet (m), but not the order with respect to hydroxide (n).
As the reaction proceeds, a violet-colored reactant will be slowly changing to a colorless product. You will measure the color change with a Vernier Colorimeter or a Vernier Spectrometer. The crystal violet solution used in this experiment has a violet color, of course, thus the Colorimeter users will be instructed to use the 565 nm (green) LED. Spectrometer users will determine an appropriate wavelength based on the absorbance spectrum of the solution. We will assume that absorbance is proportional to the concentration of crystal violet (Beer’s law). Absorbance will be used in place of concentration in plotting the following three graphs:
- Absorbance vs. time: A linear plot indicates a zero order reaction (k = –slope).
- ln Absorbance vs. time: A linear plot indicates a first order reaction (k = –slope).
- 1/Absorbance vs. time: A linear plot indicates a second order reaction (k = slope).
Once the order with respect to crystal violet has been determined, you will also be finding the rate constant, k, and the half-life for this reaction.
Objectives
In this experiment, you will
- Observe the reaction between crystal violet and sodium hydroxide.
- Monitor the absorbance of the crystal violet solution with time.
- Graph Absorbance vs. time, ln Absorbance vs. time, and 1/Absorbance vs. time.
- Determine the order of the reaction.
- Determine the rate constant, k, and the half-life for this reaction.
Sensors and Equipment
This experiment features the following sensors and equipment. Additional equipment may be required.
Option 1

Option 3

Correlations
Teaching to an educational standard? This experiment supports the standards below.
- International Baccalaureate (IB) 2025/Chemistry
- Reactivity 2.2.11—The rate constant, k, is temperature dependent and its units are determined from the overall order of the reaction.
Ready to Experiment?
Ask an Expert
Get answers to your questions about how to teach this experiment with our support team.
- Call toll-free: 888-837-6437
- Chat with Us
- Email support@vernier.com
Purchase the Lab Book
This experiment is #30 of Chemistry with Vernier. The experiment in the book includes student instructions as well as instructor information for set up, helpful hints, and sample graphs and data.



