Energy Content of Fuels
Experiment #17 from Chemistry with Vernier
- Education Level
- High School
- Subject
- Chemistry
Introduction
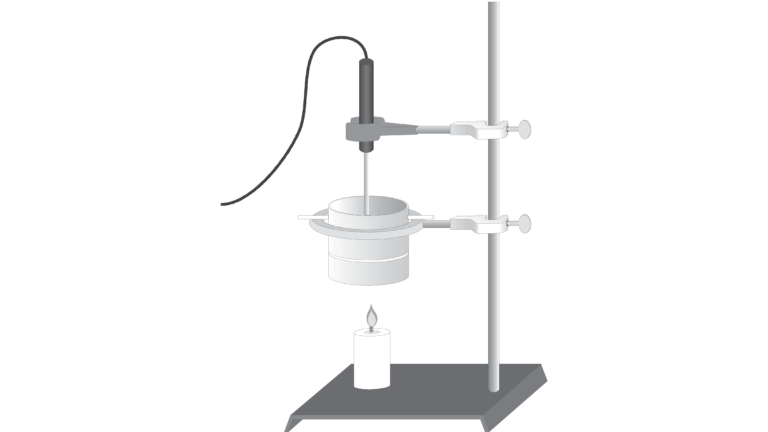
In this experiment, you will find and compare the heat of combustion of two different fuels: paraffin wax and ethanol. Paraffin is a member of a group of compounds called alkanes that are composed entirely of carbon and hydrogen atoms. Many alkanes, such as gasoline and diesel oil, are important fuels. Ethanol, C2H5OH, is used as a gasoline additive (gasohol) and as a gasoline substitute. In this experiment, you will compare the energy content of paraffin and ethanol by measuring their heats of combustion in kJ/g of fuel.
In order to find the heat of combustion, you will first use the energy from burning ethanol or paraffin to heat a known quantity of water. By monitoring the temperature of the water, you can find the amount of heat transferred to it, using the formula
where q is heat, Cp is the specific heat capacity of water, m is the mass of water, and Δt is the change in temperature of the water. Finally, the amount of fuel burned will be taken into account by calculating the heat per gram of fuel consumed in the combustion.
Objectives
In this experiment, you will
- Compare the heat of combustion for paraffin wax and ethanol.
- Calculate the heat of combustion and percent efficiency for both fuels.
Sensors and Equipment
This experiment features the following sensors and equipment. Additional equipment may be required.
Option 1

Correlations
Teaching to an educational standard? This experiment supports the standards below.
- International Baccalaureate (IB) 2025/Chemistry
- Reactivity 1.2.3—Standard enthalpy changes of combustion, ΔHc⦵, and formation, ΔHf⦵, data are used in thermodynamic calculations.
Ready to Experiment?
Ask an Expert
Get answers to your questions about how to teach this experiment with our support team.
- Call toll-free: 888-837-6437
- Chat with Us
- Email support@vernier.com
Purchase the Lab Book
This experiment is #17 of Chemistry with Vernier. The experiment in the book includes student instructions as well as instructor information for set up, helpful hints, and sample graphs and data.


