Vapor Pressure of Liquids
Experiment #10 from Chemistry with Vernier
- Education Level
- High School
- Subject
- Chemistry
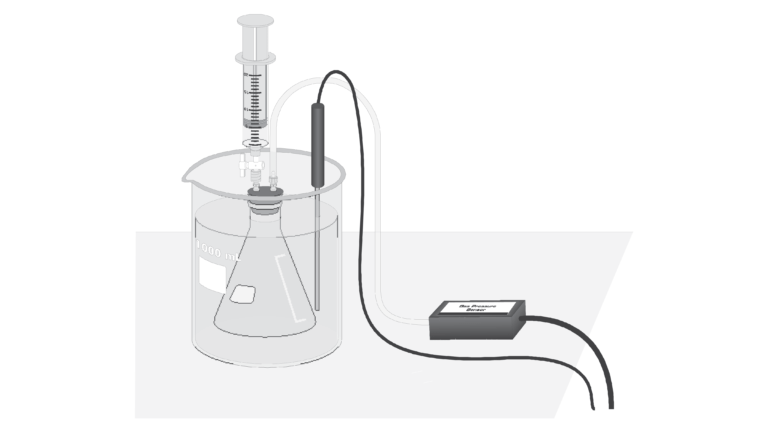
Introduction
In this experiment, you will investigate the relationship between the vapor pressure of a liquid and its temperature. When a liquid is added to the Erlenmeyer flask, it will evaporate into the air above it in the flask. Eventually, equilibrium is reached between the rate of evaporation and the rate of condensation. At this point, the vapor pressure of the liquid is equal to the partial pressure of its vapor in the flask. Pressure and temperature data will be collected using a Gas Pressure Sensor and a Temperature Probe. The flask will be placed in water baths of different temperatures to determine the effect of temperature on vapor pressure. You will also compare the vapor pressure of two different liquids, ethanol and methanol, at the same temperature.
Objectives
In this experiment, you will
- Investigate the relationship between the vapor pressure of a liquid and its temperature.
- Compare the vapor pressure of two different liquids at the same temperature.
Sensors and Equipment
This experiment features the following sensors and equipment. Additional equipment may be required.
Ready to Experiment?
Ask an Expert
Get answers to your questions about how to teach this experiment with our support team.
- Call toll-free: 888-837-6437
- Chat with Us
- Email support@vernier.com
Purchase the Lab Book
This experiment is #10 of Chemistry with Vernier. The experiment in the book includes student instructions as well as instructor information for set up, helpful hints, and sample graphs and data.





