Introduction
The heat absorbed or released during a chemical reaction is equal to the enthalpy change (ΔH) for the reaction, at constant pressure. Calorimetry is the measurement of heat absorbed or released during chemical and physical processes.
Objectives
In the Preliminary Activity, you will gain experience using a Temperature Probe and a calorimeter as you determine the enthalpy change as a hydrochloric acid solution is neutralized by a solution of sodium hydroxide.
After completing the Preliminary Activity, you will first use reference sources to find out more about heat, enthalpy, enthalpy changes, and calorimetry before you choose and investigate a researchable question involving enthalpy changes.
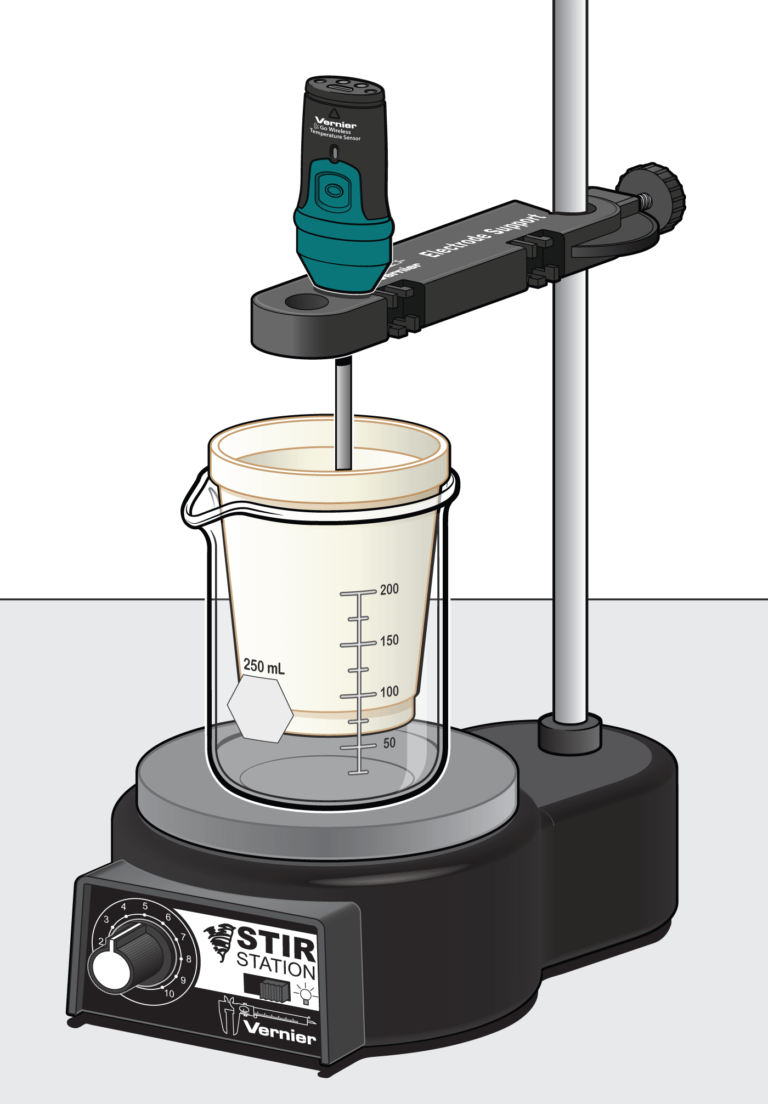
Sensors and Equipment
This experiment features the following sensors and equipment. Additional equipment may be required.
Option 1

Ready to Experiment?
Ask an Expert
Get answers to your questions about how to teach this experiment with our support team.
- Call toll-free: 888-837-6437
- Chat with Us
- Email support@vernier.com
Purchase the Lab Book
This experiment is #9 of Investigating Chemistry through Inquiry. The experiment in the book includes student instructions as well as instructor information for set up, helpful hints, and sample graphs and data.


