Introduction
Enzymes are typically proteins that act as catalysts, speeding up chemical reactions that would take far too long to occur on their own. Enzymes speed up the vast majority of the chemical reactions that occur in cells. Reactions that break down molecules (such as those involved in digestion and cellular respiration) and those that build up molecules (such as the ones involved in photosynthesis and DNA replication) all require enzymes. Each type of enzyme has a specific shape that compliments the structure of its substrate. The substrate is the molecule or molecules that the enzyme converts into product.
In this investigation, you will be studying cellobiase. Cellobiase is involved in the last step of the process of breaking down cellulose, a molecule made up of long chains of glucose that are found in plant cell walls, into glucose. This is a natural process that is used by many fungi as well as bacteria (such as those found in termite guts, the stomachs of ruminants, and compost) to produce glucose as a food source. Breaking down the cellulose from plants into sugar is also an important step in the creation of ethanol for fuel.
The natural substrate for the enzyme cellobiase is cellobiose. This is a disaccharide composed of two beta glucose molecules. However, when scientists study enzyme function, it is best if there is an easy way to detect either the amount of substrate that is used up or the amount of product that is formed. Solutions of cellobiose (substrate) and glucose (product) are clear, and there are not many simple, inexpensive, fast methods to detect these molecules quantitatively.
To make this reaction easier to follow, an artificial substrate, p-nitrophenyl glucopyranoside, will be used. This artificial substrate can also bind to the enzyme and be broken down in a manner similar to the natural substrate cellobiose. When the artificial substrate, p-nitrophenyl glucopyranoside, is broken down by cellobiase, it produces glucose and p-nitrophenol. When p-nitrophenol is mixed with a basic solution referred to as the stop solution, it will stop the reaction and turn the solution yellow. The amount of yellow color is proportional to the amount of p-nitrophenol present. For every molecule of p-nitrophenol present, one molecule of p‑nitrophenyl glucopyranoside is broken apart. For the cellobiase reactions being run, another advantage of using a basic solution to develop the color of the p-nitrophenol is that the basic pH will also denature the enzyme and stop the reaction.
Objectives
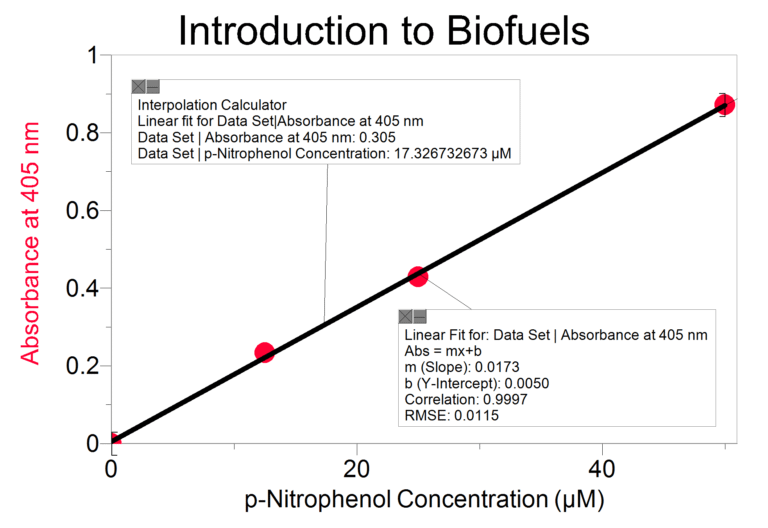
In this Preliminary Activity, you will use a spectrophotometer to determine p-nitrophenol concentration. You will first produce a p-nitrophenol containing sample with the reaction. Next, you will determine the absorbance values for a set of p-nitrophenol solutions with known concentrations in order to create a standard curve. When a graph of absorbance vs. concentration is plotted for the standard solutions, a direct relationship should result. The direct relationship between absorbance and concentration for a solution is known as Beer’s law. You will then determine the p-nitrophenol concentration of your prepared sample by measuring its absorbance and then finding the corresponding concentration on the standard curve. Because the relationship is linear, you could also calculate the
p-nitrophenol concentration using the formula for the standard curve. You will also determine the initial rate of your p-nitrophenol producing reaction.
After completing the Preliminary Activity, you will first use reference sources to find out more about biofuels and the cellobiase catalyzed reaction that produces p-nitrophenol before you choose and investigate a researchable question. Some topics to consider in your reference search are:
- catalyst
- enzyme
- biofuel
- ethanol
- cellulose
- cellobiose
- cellobiase
- substrate
- p-nitrophenol
- Beer’s law
Sensors and Equipment
This experiment features the following sensors and equipment. Additional equipment may be required.
Correlations
Teaching to an educational standard? This experiment supports the standards below.
- International Baccalaureate (IB) 2025/Biology
- B1.1.6—Structure of cellulose related to its function as a structural polysaccharide in plants
Ready to Experiment?
Ask an Expert
Get answers to your questions about how to teach this experiment with our support team.
- Call toll-free: 888-837-6437
- Chat with Us
- Email support@vernier.com
Purchase the Lab Book
This experiment is #7 of Investigating Biology through Inquiry. The experiment in the book includes student instructions as well as instructor information for set up, helpful hints, and sample graphs and data.



