Introduction
Organisms are often very sensitive to the effect of acids and bases in their environment. They need to maintain a stable internal pH in order to survive—even in the event of environmental changes. Many naturally occurring biological, geological, and human-made chemicals are capable of stabilizing the environment’s pH in a process called buffering. This may allow organisms to better survive in diverse environments found throughout the earth.
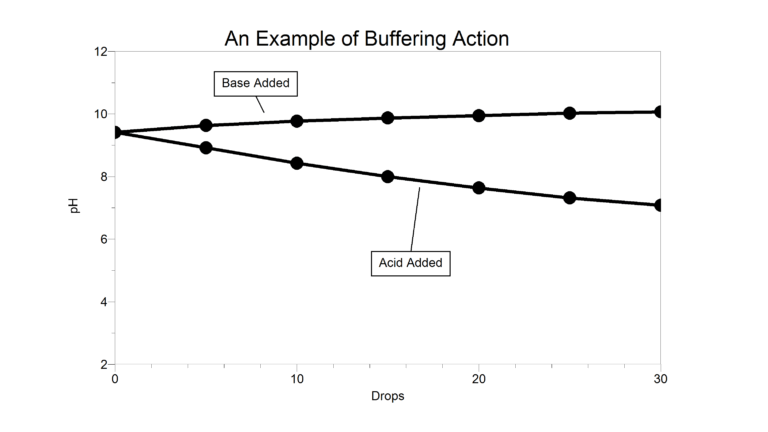
A buffer is a mixture of a weak acid and its conjugate base, or a weak base and its conjugate acid. A buffer’s function is to absorb acids (H+ or H3O+ ions) or bases (OH– ions) so that pH changes very, very little. The bicarbonate-carbon dioxide buffer system, for example, keeps the pH of blood in humans between 7.3 and 7.5.
Baking soda, like many substances, has some buffering ability. In the Preliminary Activity, you will determine the buffering ability of a baking soda solution. You will first use a pH Sensor to monitor pH as you add hydrochloric acid (HCl) to one portion of the sample. Subsequently, you will monitor pH as you add basic sodium hydroxide (NaOH) solution to another portion of the sample. When data collection is complete, you will determine the total pH change of the baking soda sample.
Sensors and Equipment
This experiment features the following sensors and equipment. Additional equipment may be required.
Option 1

Option 2

Ready to Experiment?
Ask an Expert
Get answers to your questions about how to teach this experiment with our support team.
- Call toll-free: 888-837-6437
- Chat with Us
- Email support@vernier.com
Purchase the Lab Book
This experiment is #1 of Investigating Biology through Inquiry. The experiment in the book includes student instructions as well as instructor information for set up, helpful hints, and sample graphs and data.

