To get accurate results, make sure you are doing the following:
1) The user manual states that you need at least 2 cm of solution, which is equivalent to about 5 mL. See What is the sample volume required by your Polarimeter?
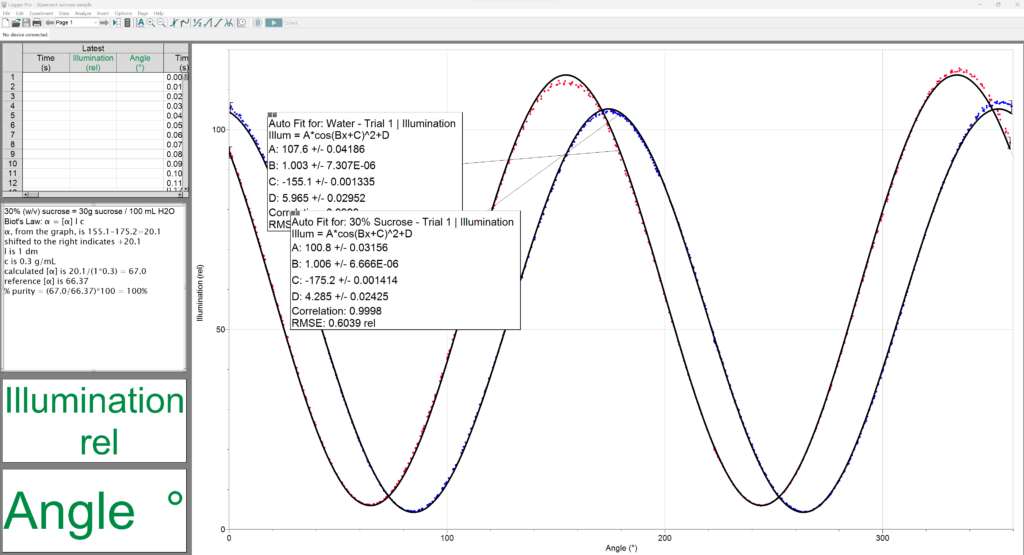
2) Often times people prepare the sample incorrectly. A 30% solution of sucrose should be 30 g sucrose diluted to a total volume of 100 mL.
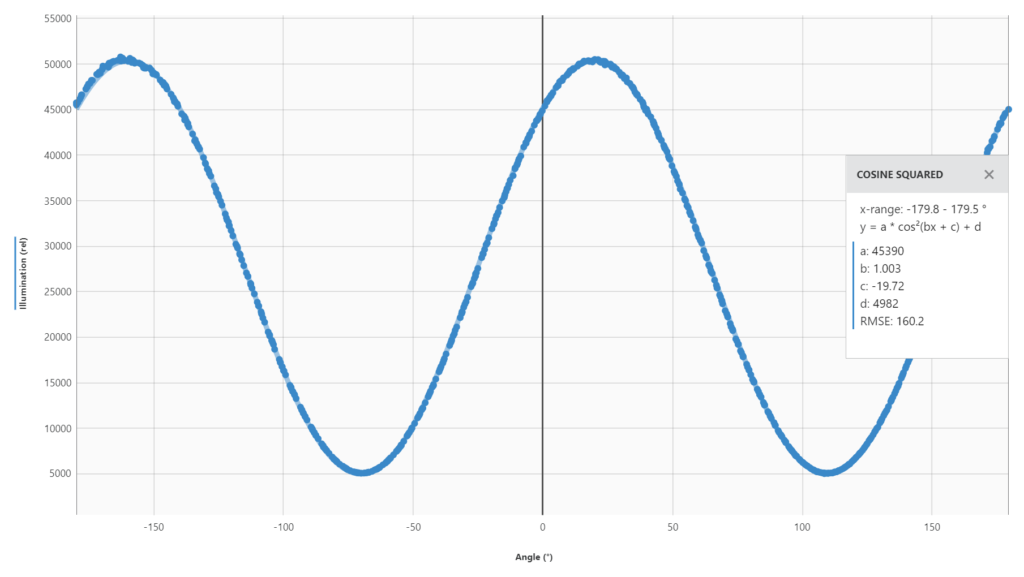
3) Keep in mind that there are two peaks for every sample on the graph, because we are showing all 360 degrees on the x-axis. So, there will be a peak every 180 degrees. You have to pick the water peak and the sucrose peak that make sense to relate to each other. Typically, this means the two peaks that are closest together. We know that sucrose is a positive rotator, so it will appear to the right of the water peak. (Whereas if you use fructose, for example, it will appear to the left of water.)
You can also do some quick math:
The observed specific rotation under the conditions stated here should be close to: literature specific rotation x optical path (dm) x concentration (g/mL) In the example of a 30% sucrose solution at 1 dm path length:
66.4 degrees x 1.0 dm x 0.3 g/mL = 19.9 degrees.
Therefore, I would expect the sucrose peak to be about 20 degrees to the right of the water peak.
Please review the user manual (CHEM-POL Specifications and User Guide; GDX-POL Specifications and User Guide) for additional tips.
We also offer free experiments with sample data to help you get started: Do you have experiments written for the Polarimeter?
Also see:
Chemical Polarimeter Troubleshooting and FAQs
I'm using a LabPro as my data collection interface for the Chemical Polarimeter. Do I need to follow any special instructions?
Go Direct Polarimeter Troubleshooting and FAQs