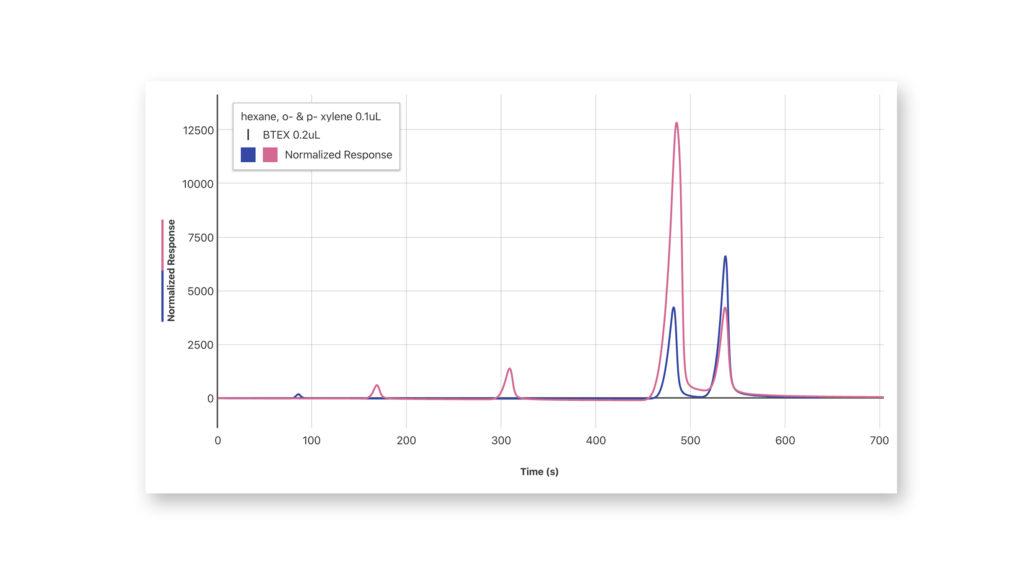
Using a Gas Chromatograph: Identifying an Unknown Compound
There are many different types of chromatography: paper, thin layer (TLC), liquid (LC), high-pressure liquid (HPLC), and gas (GC). Chromatography is applied in many fields. Biochemists use liquid chromatography to separate proteins; chemists use GC, TLC, and HPLC to identify organic compounds. Forensic scientists and other specialties use gas chromatography for drug tests, toxin screens, and environmental analysis.
All types of chromatography use the same principles that include a stationary phase and a mobile phase. The stationary phase is immobile on the column or the plate and the mobile phase travels from a start point to an end point. Compounds travel from the start to the end at a specific rate depending on their competing affinity for the mobile gas/liquid phase versus the stationary solid phase. Compounds adhere to the stationary phase through dipole interactions, dispersion forces, or ionic interactions.