Introduction
The decomposition of hydrogen peroxide in aqueous solution proceeds very slowly. A bottle of 3% hydrogen peroxide sitting on a grocery store shelf is stable for a lengthy period. The decomposition takes place according to the reaction below.
A number of catalysts, one of which is potassium iodide, can be used to speed up this reaction.
Before data collection begins, there is no product, and the pressure is the same as atmospheric pressure. Shortly after data collection begins, oxygen accumulates at a rather constant rate. The slope of the curve at this initial time is constant and is called the initial rate. As the peroxide is decomposed, less of it is available to react and the O2 is produced at lower rates. When no more peroxide is left, O2 is no longer produced. When data collection is complete, you will perform a linear fit on the resultant graph to determine the initial reaction rate.
Objectives
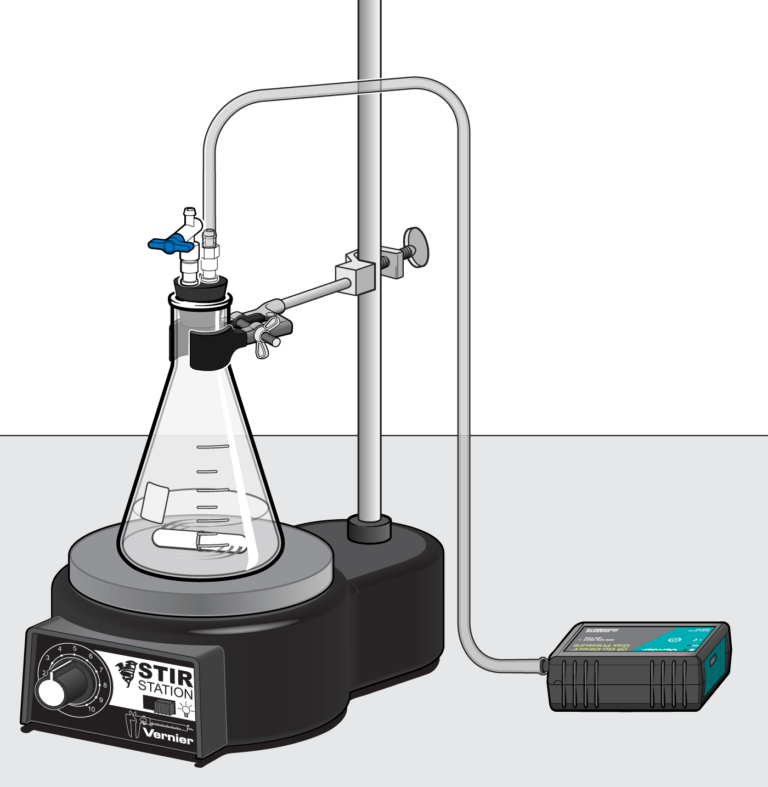
In the Preliminary Activity, you will use a Gas Pressure Sensor to monitor the pressure increase due to the production of oxygen gas inside an Erlenmeyer flask as potassium iodide catalytically decomposes hydrogen peroxide.
After completing the Preliminary Activity, you will first use reference sources to find out more about reaction rates and factors that influence them before you choose and investigate a researchable question dealing with reaction rates.
Sensors and Equipment
This experiment features the following sensors and equipment. Additional equipment may be required.
Ready to Experiment?
Ask an Expert
Get answers to your questions about how to teach this experiment with our support team.
- Call toll-free: 888-837-6437
- Chat with Us
- Email support@vernier.com
Purchase the Lab Book
This experiment is #22 of Investigating Chemistry through Inquiry. The experiment in the book includes student instructions as well as instructor information for set up, helpful hints, and sample graphs and data.





